Copper Single-Atoms Loaded on Molybdenum Disulphide Drive Bacterial Cuproptosis-Like Death and Interrupt Drug-Resistance Compensation Pathways
Corresponding Author: Xianwen Wang
Nano-Micro Letters,
Vol. 18 (2026), Article Number: 111
Abstract
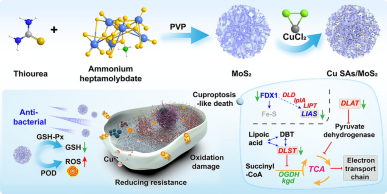
The development of highly efficient and multifunctional nanozymes holds promise for addressing the challenges posed by drug-resistant bacteria. Here, copper single-atom-loaded MoS2 nanozymes (Cu SAs/MoS2) were developed to effectively combat drug-resistant bacteria by synergistically integrating the triple strategies of oxidative damage, cuproptosis-like death and disruption of cell wall synthesis. Density functional theory revealed that each Cu center coordinated with three sulfur ligands, enhancing the adsorption of H2O2, which reduced the activation energy of the key step by 17%, thereby improving peroxidase-like (POD-like) activity. The generation of reactive oxygen species in combination with Cu SAs/MoS2 glutathione peroxidase-like (GSH-Px-like) for glutathione scavenging resulted in an imbalance in redox homeostasis within bacteria. Cu SAs/MoS2, which act as nanopioneers, drive oxidative stress to initiate the process of cuproptosis-like death, leading to abnormal aggregation of lipoylated proteins and inactivation of iron‒sulfur cluster proteins. Moreover, Cu SAs/MoS2 inhibited the biosynthesis of the peptidoglycan synthesis precursors d-glutamate and m-diaminopimelic acid and disrupted the peptidoglycan cross-linking process mediated by penicillin-binding proteins, effectively blocking the compensatory cell wall remodeling pathway of β-lactam-resistant bacteria. Overall, Cu SAs/MoS2 with multiple functions can not only efficiently kill bacteria but also decelerate the development of bacterial resistance to combat drug-resistant bacterial infections.
Highlights:
1 Peroxidase-like and glutathione peroxidase-like activities were significantly enhanced by atomic-level doping of Cu SAs/MoS2, which efficiently generated reactive oxygen species (ROS) and caused oxidative damage to drug-resistant bacteria.
2 The ROS storms generated by single-atom-loaded MoS2 nanozymes (Cu SAs/MoS2) altered bacterial membrane permeability and facilitated Cu2+ entry into bacteria, enhancing bacterial cuproptosis-like death.
3 Cu SAs/MoS2 interferes with bacterial energy metabolism and cell wall synthesis and inhibits peptidoglycan synthesis, weakening bacterial adaptation and drug resistance.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- M. Naghavi, S.E. Vollset, K.S. Ikuta, L.R. Swetschinski, A.P. Gray et al., Global burden of bacterial antimicrobial resistance 1990–2021: a systematic analysis with forecasts to 2050. Lancet 404(10459), 1199–1226 (2024). https://doi.org/10.1016/S0140-6736(24)01867-1
- C.J.L. Murray, K.S. Ikuta, F. Sharara, L. Swetschinski, G. Robles Aguilar et al., Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399(10325), 629–655 (2022). https://doi.org/10.1016/s0140-6736(21)02724-0
- T. Mestrovic, G. Robles Aguilar, L.R. Swetschinski, K.S. Ikuta, A.P. Gray et al., The burden of bacterial antimicrobial resistance in the WHO European region in 2019: a cross-country systematic analysis. Lancet Public Health 7(11), e897–e913 (2022). https://doi.org/10.1016/S2468-2667(22)00225-0
- M.J. Mitcheltree, A. Pisipati, E.A. Syroegin, K.J. Silvestre, D. Klepacki et al., A synthetic antibiotic class overcoming bacterial multidrug resistance. Nature 599(7885), 507–512 (2021). https://doi.org/10.1038/s41586-021-04045-6
- M. Stracy, O. Snitser, I. Yelin, Y. Amer, M. Parizade et al., Minimizing treatment-induced emergence of antibiotic resistance in bacterial infections. Science 375(6583), 889–894 (2022). https://doi.org/10.1126/science.abg9868
- H. Niu, J. Gu, Y. Zhang, Bacterial persisters: molecular mechanisms and therapeutic development. Signal Transduct. Target. Ther. 9(1), 174 (2024). https://doi.org/10.1038/s41392-024-01866-5
- E. Tshibangu-Kabamba, Y. Yamaoka, Helicobacter pylori infection and antibiotic resistance: from biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 18(9), 613–629 (2021). https://doi.org/10.1038/s41575-021-00449-x
- M. Zabiszak, J. Frymark, K. Ogawa, M. Skrobańska, M. Nowak et al., Complexes of β-lactam antibiotics and their Schiff-base derivatives as a weapon in the fight against bacterial resistance. Coord. Chem. Rev. 493, 215326 (2023). https://doi.org/10.1016/j.ccr.2023.215326
- A.F. Adedeji-Olulana, K. Wacnik, L. Lafage, L. Pasquina-Lemonche, M. Tinajero-Trejo et al., Two codependent routes lead to high-level MRSA. Science 386(6721), 573–580 (2024). https://doi.org/10.1126/science.adn1369
- J.F. Fisher, S. Mobashery, Β-lactams against the fortress of the gram-positive Staphylococcus aureus bacterium. Chem. Rev. 121(6), 3412–3463 (2021). https://doi.org/10.1021/acs.chemrev.0c01010
- Y. Chen, Q. Liu, H.E. Takiff, Q. Gao, Comprehensive genomic analysis of Mycobacterium tuberculosis reveals limited impact of high-fitness genotypes on MDR-TB transmission. J. Infect. 85(1), 49–56 (2022). https://doi.org/10.1016/j.jinf.2022.05.012
- W.P.J. Smith, B.R. Wucher, C.D. Nadell, K.R. Foster, Bacterial defences: mechanisms, evolution and antimicrobial resistance. Nat. Rev. Microbiol. 21(8), 519–534 (2023). https://doi.org/10.1038/s41579-023-00877-3
- S.M. Gygli, C. Loiseau, L. Jugheli, N. Adamia, A. Trauner et al., Prisons as ecological drivers of fitness-compensated multidrug-resistant Mycobacterium tuberculosis. Nat. Med. 27(7), 1171–1177 (2021). https://doi.org/10.1038/s41591-021-01358-x
- A. Alonso-Del Valle, R. León-Sampedro, J. Rodríguez-Beltrán, J. DelaFuente, M. Hernández-García et al., Variability of plasmid fitness effects contributes to plasmid persistence in bacterial communities. Nat. Commun. 12(1), 2653 (2021). https://doi.org/10.1038/s41467-021-22849-y
- F. Coll, B. Blane, K.L. Bellis, M. Matuszewska, T. Wonfor et al., The mutational landscape of Staphylococcus aureus during colonisation. Nat. Commun. 16, 302 (2025). https://doi.org/10.1038/s41467-024-55186-x
- J.M.V. Makabenta, A. Nabawy, C.-H. Li, S. Schmidt-Malan, R. Patel et al., Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 19(1), 23–36 (2021). https://doi.org/10.1038/s41579-020-0420-1
- P.P. Kalelkar, M. Riddick, A.J. García, Biomaterial-based antimicrobial therapies for the treatment of bacterial infections. Nat. Rev. Mater. 7(1), 39–54 (2022). https://doi.org/10.1038/s41578-021-00362-4
- L. Jin, F. Cao, Y. Gao, C. Zhang, Z. Qian et al., Microenvironment-activated nanozyme-armed bacteriophages efficiently combat bacterial infection. Adv. Mater. 35(30), e2301349 (2023). https://doi.org/10.1002/adma.202301349
- W. Wang, P. Gao, H. Gui, X. Wei, H. Zhang et al., Copper-based nanomaterials for the treatment of bacteria-infected wounds: material classification, strategies and mechanisms. Coord. Chem. Rev. 522, 216205 (2025). https://doi.org/10.1016/j.ccr.2024.216205
- D. Jiang, D. Ni, Z.T. Rosenkrans, P. Huang, X. Yan et al., Nanozyme: new horizons for responsive biomedical applications. Chem. Soc. Rev. 48(14), 3683–3704 (2019). https://doi.org/10.1039/c8cs00718g
- B. Xu, Y. Cui, W. Wang, S. Li, C. Lyu et al., Immunomodulation-enhanced nanozyme-based tumor catalytic therapy. Adv. Mater. 32(33), e2003563 (2020). https://doi.org/10.1002/adma.202003563
- L. Wang, F. Gao, A. Wang, X. Chen, H. Li et al., Defect-rich adhesive molybdenum disulfide/rGO vertical heterostructures with enhanced nanozyme activity for smart bacterial killing application. Adv. Mater. 32(48), 2005423 (2020). https://doi.org/10.1002/adma.202005423
- W. Zhang, J. Liu, X. Li, Y. Zheng, L. Chen et al., Precise chemodynamic therapy of cancer by trifunctional bacterium-based nanozymes. ACS Nano 15(12), 19321–19333 (2021). https://doi.org/10.1021/acsnano.1c05605
- W. Zhu, J. Mei, X. Zhang, J. Zhou, D. Xu et al., Photothermal nanozyme-based microneedle patch against refractory bacterial biofilm infection via iron-actuated Janus ion therapy. Adv. Mater. 34(51), 2207961 (2022). https://doi.org/10.1002/adma.202207961
- C. Cao, T. Zhang, N. Yang, X. Niu, Z. Zhou et al., Pod nanozyme optimized by charge separation engineering for light/pH activated bacteria catalytic/photodynamic therapy. Signal Transduct. Target. Ther. 7(1), 86 (2022). https://doi.org/10.1038/s41392-022-00900-8
- M. Yang, Y. Liu, L. Zhang, Y. Qian, N. Li et al., Highly conjugated nanozyme with non coordination saturation for cascaded enhanced POD reaction driving antibacterial therapy. Adv. Funct. Mater. 34(42), 2404894 (2024). https://doi.org/10.1002/adfm.202404894
- C. Zhou, Q. Wang, H. Cao, J. Jiang, L. Gao, Nanozybiotics: advancing antimicrobial strategies through biomimetic mechanisms. Adv. Mater. 36(33), e2403362 (2024). https://doi.org/10.1002/adma.202403362
- X. Wang, Q. Shi, Z. Zha, D. Zhu, L. Zheng et al., Copper single-atom catalysts with photothermal performance and enhanced nanozyme activity for bacteria-infected wound therapy. Bioact. Mater. 6(12), 4389–4401 (2021). https://doi.org/10.1016/j.bioactmat.2021.04.024
- F. Wu, J. Ma, Y. Wang, L. Xie, X. Yan et al., Single copper atom photocatalyst powers an integrated catalytic cascade for drug-resistant bacteria elimination. ACS Nano 17(3), 2980–2991 (2023). https://doi.org/10.1021/acsnano.2c11550
- L. Jin, X. Liu, Y. Zheng, Z. Li, Y. Zhang et al., Interface polarization strengthened microwave catalysis of MoS2/FeS/Rhein for the therapy of bacteria-infected osteomyelitis. Adv. Funct. Mater. 32(33), 2204437 (2022). https://doi.org/10.1002/adfm.202204437
- Y.-H. Kuo, M.-C. Hsu, W.-J. Wang, H.-H. Peng, W.-P. Li, Highly conductive riboflavin-based carbon quantum dot–embedded SiO2@MoS2 nanocomposite for enhancing bioelectricity generation through synergistic direct and indirect electron transport. Nano Energy 121, 109251 (2024). https://doi.org/10.1016/j.nanoen.2023.109251
- X. Fan, Y. Gao, F. Yang, J.L. Low, L. Wang et al., A copper single-atom cascade bionanocatalyst for treating multidrug-resistant bacterial diabetic ulcer. Adv. Funct. Mater. 33(33), 2301986 (2023). https://doi.org/10.1002/adfm.202301986
- H. Yang, Y. Wu, G. Li, Q. Lin, Q. Hu et al., Scalable production of efficient single-atom copper decorated carbon membranes for CO2 electroreduction to methanol. J. Am. Chem. Soc. 141(32), 12717–12723 (2019). https://doi.org/10.1021/jacs.9b04907
- J. Li, J. Li, Y. Chen, P. Tai, P. Fu et al., Molybdenum disulfide-supported cuprous oxide nanocomposite for near-infrared-I light-responsive synergistic antibacterial therapy. ACS Nano 18(25), 16184–16198 (2024). https://doi.org/10.1021/acsnano.4c01452
- X. Ding, Y. Yu, W. Li, Y. Zhao, In situ 3D-bioprinting MoS2 accelerated gelling hydrogel scaffold for promoting chronic diabetic wound healing. Matter 6(3), 1000–1014 (2023). https://doi.org/10.1016/j.matt.2023.01.001
- Y. Huang, X. Wan, Q. Su, C. Zhao, J. Cao et al., Ultrasound-activated piezo-hot carriers trigger tandem catalysis coordinating cuproptosis-like bacterial death against implant infections. Nat. Commun. 15(1), 1643 (2024). https://doi.org/10.1038/s41467-024-45619-y
- S. Li, Y. Yue, W. Wang, M. Han, X. Wan et al., Ultrasound-activated probiotics vesicles coating for titanium implant infections through bacterial cuproptosis-like death and immunoregulation. Adv. Mater. 36(44), 2405953 (2024). https://doi.org/10.1002/adma.202405953
- W. Wang, Y. Cui, X. Wei, Y. Zang, X. Chen et al., CuCo2O4 nanoflowers with multiple enzyme activities for treating bacterium-infected wounds via cuproptosis-like death. ACS Nano 18(24), 15845–15863 (2024). https://doi.org/10.1021/acsnano.4c02825
- Z. Luo, R. Lu, T. Shi, Z. Ruan, W. Wang et al., Enhanced bacterial cuproptosis-like death via reversal of hypoxia microenvironment for biofilm infection treatment. Adv. Sci. 11(19), 2308850 (2024). https://doi.org/10.1002/advs.202308850
- Y. Xue, L. Zhang, J. Zhou, J. Chen, Y. Ma et al., Low-dose Cu ions assisted by mild thermal stimulus inducing bacterial cuproptosis-like death for antibiosis and biointegration. Adv. Funct. Mater. 34(1), 2308197 (2024). https://doi.org/10.1002/adfm.202308197
- P. Manivasagan, T. Thambi, A. Joe, H.-W. Han, S.-H. Seo et al., Progress in nanomaterial-based synergistic photothermal-enhanced chemodynamic therapy in combating bacterial infections. Prog. Mater. Sci. 144, 101292 (2024). https://doi.org/10.1016/j.pmatsci.2024.101292
- W. Wang, W. Yu, G. Li, H. Huang, X. Song et al., Engineering versatile nano-bacteria hybrids for efficient tumor therapy. Coord. Chem. Rev. 488, 215178 (2023). https://doi.org/10.1016/j.ccr.2023.215178
- X. He, Y. Lv, Y. Lin, H. Yu, Y. Zhang et al., Platinum nanops regulated V2C MXene nanoplatforms with NIR-II enhanced nanozyme effect for photothermal and chemodynamic anti-infective therapy. Adv. Mater. 36(25), 2400366 (2024). https://doi.org/10.1002/adma.202400366
- G. Kresse, J. Hafner, Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B 48(17), 13115–13118 (1993). https://doi.org/10.1103/physrevb.48.13115
- J.P. Perdew, K. Burke, M. Ernzerhof, Generalized gradient approximation made simple. Phys. Rev. Lett. 77(18), 3865–3868 (1996). https://doi.org/10.1103/physrevlett.77.3865
- P.E. Blöchl, Projector augmented-wave method. Phys. Rev. B 50(24), 17953–17979 (1994). https://doi.org/10.1103/physrevb.50.17953
- M. Zhang, Y. Wang, C. Miao, S. Lin, Y. Zheng et al., Dextran guanidinylated carbon dots with antibacterial and immunomodulatory activities as eye drops for the topical treatment of MRSA-induced infectious keratitis. Acta Biomater. 200, 591–609 (2025). https://doi.org/10.1016/j.actbio.2025.05.032
References
M. Naghavi, S.E. Vollset, K.S. Ikuta, L.R. Swetschinski, A.P. Gray et al., Global burden of bacterial antimicrobial resistance 1990–2021: a systematic analysis with forecasts to 2050. Lancet 404(10459), 1199–1226 (2024). https://doi.org/10.1016/S0140-6736(24)01867-1
C.J.L. Murray, K.S. Ikuta, F. Sharara, L. Swetschinski, G. Robles Aguilar et al., Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399(10325), 629–655 (2022). https://doi.org/10.1016/s0140-6736(21)02724-0
T. Mestrovic, G. Robles Aguilar, L.R. Swetschinski, K.S. Ikuta, A.P. Gray et al., The burden of bacterial antimicrobial resistance in the WHO European region in 2019: a cross-country systematic analysis. Lancet Public Health 7(11), e897–e913 (2022). https://doi.org/10.1016/S2468-2667(22)00225-0
M.J. Mitcheltree, A. Pisipati, E.A. Syroegin, K.J. Silvestre, D. Klepacki et al., A synthetic antibiotic class overcoming bacterial multidrug resistance. Nature 599(7885), 507–512 (2021). https://doi.org/10.1038/s41586-021-04045-6
M. Stracy, O. Snitser, I. Yelin, Y. Amer, M. Parizade et al., Minimizing treatment-induced emergence of antibiotic resistance in bacterial infections. Science 375(6583), 889–894 (2022). https://doi.org/10.1126/science.abg9868
H. Niu, J. Gu, Y. Zhang, Bacterial persisters: molecular mechanisms and therapeutic development. Signal Transduct. Target. Ther. 9(1), 174 (2024). https://doi.org/10.1038/s41392-024-01866-5
E. Tshibangu-Kabamba, Y. Yamaoka, Helicobacter pylori infection and antibiotic resistance: from biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 18(9), 613–629 (2021). https://doi.org/10.1038/s41575-021-00449-x
M. Zabiszak, J. Frymark, K. Ogawa, M. Skrobańska, M. Nowak et al., Complexes of β-lactam antibiotics and their Schiff-base derivatives as a weapon in the fight against bacterial resistance. Coord. Chem. Rev. 493, 215326 (2023). https://doi.org/10.1016/j.ccr.2023.215326
A.F. Adedeji-Olulana, K. Wacnik, L. Lafage, L. Pasquina-Lemonche, M. Tinajero-Trejo et al., Two codependent routes lead to high-level MRSA. Science 386(6721), 573–580 (2024). https://doi.org/10.1126/science.adn1369
J.F. Fisher, S. Mobashery, Β-lactams against the fortress of the gram-positive Staphylococcus aureus bacterium. Chem. Rev. 121(6), 3412–3463 (2021). https://doi.org/10.1021/acs.chemrev.0c01010
Y. Chen, Q. Liu, H.E. Takiff, Q. Gao, Comprehensive genomic analysis of Mycobacterium tuberculosis reveals limited impact of high-fitness genotypes on MDR-TB transmission. J. Infect. 85(1), 49–56 (2022). https://doi.org/10.1016/j.jinf.2022.05.012
W.P.J. Smith, B.R. Wucher, C.D. Nadell, K.R. Foster, Bacterial defences: mechanisms, evolution and antimicrobial resistance. Nat. Rev. Microbiol. 21(8), 519–534 (2023). https://doi.org/10.1038/s41579-023-00877-3
S.M. Gygli, C. Loiseau, L. Jugheli, N. Adamia, A. Trauner et al., Prisons as ecological drivers of fitness-compensated multidrug-resistant Mycobacterium tuberculosis. Nat. Med. 27(7), 1171–1177 (2021). https://doi.org/10.1038/s41591-021-01358-x
A. Alonso-Del Valle, R. León-Sampedro, J. Rodríguez-Beltrán, J. DelaFuente, M. Hernández-García et al., Variability of plasmid fitness effects contributes to plasmid persistence in bacterial communities. Nat. Commun. 12(1), 2653 (2021). https://doi.org/10.1038/s41467-021-22849-y
F. Coll, B. Blane, K.L. Bellis, M. Matuszewska, T. Wonfor et al., The mutational landscape of Staphylococcus aureus during colonisation. Nat. Commun. 16, 302 (2025). https://doi.org/10.1038/s41467-024-55186-x
J.M.V. Makabenta, A. Nabawy, C.-H. Li, S. Schmidt-Malan, R. Patel et al., Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 19(1), 23–36 (2021). https://doi.org/10.1038/s41579-020-0420-1
P.P. Kalelkar, M. Riddick, A.J. García, Biomaterial-based antimicrobial therapies for the treatment of bacterial infections. Nat. Rev. Mater. 7(1), 39–54 (2022). https://doi.org/10.1038/s41578-021-00362-4
L. Jin, F. Cao, Y. Gao, C. Zhang, Z. Qian et al., Microenvironment-activated nanozyme-armed bacteriophages efficiently combat bacterial infection. Adv. Mater. 35(30), e2301349 (2023). https://doi.org/10.1002/adma.202301349
W. Wang, P. Gao, H. Gui, X. Wei, H. Zhang et al., Copper-based nanomaterials for the treatment of bacteria-infected wounds: material classification, strategies and mechanisms. Coord. Chem. Rev. 522, 216205 (2025). https://doi.org/10.1016/j.ccr.2024.216205
D. Jiang, D. Ni, Z.T. Rosenkrans, P. Huang, X. Yan et al., Nanozyme: new horizons for responsive biomedical applications. Chem. Soc. Rev. 48(14), 3683–3704 (2019). https://doi.org/10.1039/c8cs00718g
B. Xu, Y. Cui, W. Wang, S. Li, C. Lyu et al., Immunomodulation-enhanced nanozyme-based tumor catalytic therapy. Adv. Mater. 32(33), e2003563 (2020). https://doi.org/10.1002/adma.202003563
L. Wang, F. Gao, A. Wang, X. Chen, H. Li et al., Defect-rich adhesive molybdenum disulfide/rGO vertical heterostructures with enhanced nanozyme activity for smart bacterial killing application. Adv. Mater. 32(48), 2005423 (2020). https://doi.org/10.1002/adma.202005423
W. Zhang, J. Liu, X. Li, Y. Zheng, L. Chen et al., Precise chemodynamic therapy of cancer by trifunctional bacterium-based nanozymes. ACS Nano 15(12), 19321–19333 (2021). https://doi.org/10.1021/acsnano.1c05605
W. Zhu, J. Mei, X. Zhang, J. Zhou, D. Xu et al., Photothermal nanozyme-based microneedle patch against refractory bacterial biofilm infection via iron-actuated Janus ion therapy. Adv. Mater. 34(51), 2207961 (2022). https://doi.org/10.1002/adma.202207961
C. Cao, T. Zhang, N. Yang, X. Niu, Z. Zhou et al., Pod nanozyme optimized by charge separation engineering for light/pH activated bacteria catalytic/photodynamic therapy. Signal Transduct. Target. Ther. 7(1), 86 (2022). https://doi.org/10.1038/s41392-022-00900-8
M. Yang, Y. Liu, L. Zhang, Y. Qian, N. Li et al., Highly conjugated nanozyme with non coordination saturation for cascaded enhanced POD reaction driving antibacterial therapy. Adv. Funct. Mater. 34(42), 2404894 (2024). https://doi.org/10.1002/adfm.202404894
C. Zhou, Q. Wang, H. Cao, J. Jiang, L. Gao, Nanozybiotics: advancing antimicrobial strategies through biomimetic mechanisms. Adv. Mater. 36(33), e2403362 (2024). https://doi.org/10.1002/adma.202403362
X. Wang, Q. Shi, Z. Zha, D. Zhu, L. Zheng et al., Copper single-atom catalysts with photothermal performance and enhanced nanozyme activity for bacteria-infected wound therapy. Bioact. Mater. 6(12), 4389–4401 (2021). https://doi.org/10.1016/j.bioactmat.2021.04.024
F. Wu, J. Ma, Y. Wang, L. Xie, X. Yan et al., Single copper atom photocatalyst powers an integrated catalytic cascade for drug-resistant bacteria elimination. ACS Nano 17(3), 2980–2991 (2023). https://doi.org/10.1021/acsnano.2c11550
L. Jin, X. Liu, Y. Zheng, Z. Li, Y. Zhang et al., Interface polarization strengthened microwave catalysis of MoS2/FeS/Rhein for the therapy of bacteria-infected osteomyelitis. Adv. Funct. Mater. 32(33), 2204437 (2022). https://doi.org/10.1002/adfm.202204437
Y.-H. Kuo, M.-C. Hsu, W.-J. Wang, H.-H. Peng, W.-P. Li, Highly conductive riboflavin-based carbon quantum dot–embedded SiO2@MoS2 nanocomposite for enhancing bioelectricity generation through synergistic direct and indirect electron transport. Nano Energy 121, 109251 (2024). https://doi.org/10.1016/j.nanoen.2023.109251
X. Fan, Y. Gao, F. Yang, J.L. Low, L. Wang et al., A copper single-atom cascade bionanocatalyst for treating multidrug-resistant bacterial diabetic ulcer. Adv. Funct. Mater. 33(33), 2301986 (2023). https://doi.org/10.1002/adfm.202301986
H. Yang, Y. Wu, G. Li, Q. Lin, Q. Hu et al., Scalable production of efficient single-atom copper decorated carbon membranes for CO2 electroreduction to methanol. J. Am. Chem. Soc. 141(32), 12717–12723 (2019). https://doi.org/10.1021/jacs.9b04907
J. Li, J. Li, Y. Chen, P. Tai, P. Fu et al., Molybdenum disulfide-supported cuprous oxide nanocomposite for near-infrared-I light-responsive synergistic antibacterial therapy. ACS Nano 18(25), 16184–16198 (2024). https://doi.org/10.1021/acsnano.4c01452
X. Ding, Y. Yu, W. Li, Y. Zhao, In situ 3D-bioprinting MoS2 accelerated gelling hydrogel scaffold for promoting chronic diabetic wound healing. Matter 6(3), 1000–1014 (2023). https://doi.org/10.1016/j.matt.2023.01.001
Y. Huang, X. Wan, Q. Su, C. Zhao, J. Cao et al., Ultrasound-activated piezo-hot carriers trigger tandem catalysis coordinating cuproptosis-like bacterial death against implant infections. Nat. Commun. 15(1), 1643 (2024). https://doi.org/10.1038/s41467-024-45619-y
S. Li, Y. Yue, W. Wang, M. Han, X. Wan et al., Ultrasound-activated probiotics vesicles coating for titanium implant infections through bacterial cuproptosis-like death and immunoregulation. Adv. Mater. 36(44), 2405953 (2024). https://doi.org/10.1002/adma.202405953
W. Wang, Y. Cui, X. Wei, Y. Zang, X. Chen et al., CuCo2O4 nanoflowers with multiple enzyme activities for treating bacterium-infected wounds via cuproptosis-like death. ACS Nano 18(24), 15845–15863 (2024). https://doi.org/10.1021/acsnano.4c02825
Z. Luo, R. Lu, T. Shi, Z. Ruan, W. Wang et al., Enhanced bacterial cuproptosis-like death via reversal of hypoxia microenvironment for biofilm infection treatment. Adv. Sci. 11(19), 2308850 (2024). https://doi.org/10.1002/advs.202308850
Y. Xue, L. Zhang, J. Zhou, J. Chen, Y. Ma et al., Low-dose Cu ions assisted by mild thermal stimulus inducing bacterial cuproptosis-like death for antibiosis and biointegration. Adv. Funct. Mater. 34(1), 2308197 (2024). https://doi.org/10.1002/adfm.202308197
P. Manivasagan, T. Thambi, A. Joe, H.-W. Han, S.-H. Seo et al., Progress in nanomaterial-based synergistic photothermal-enhanced chemodynamic therapy in combating bacterial infections. Prog. Mater. Sci. 144, 101292 (2024). https://doi.org/10.1016/j.pmatsci.2024.101292
W. Wang, W. Yu, G. Li, H. Huang, X. Song et al., Engineering versatile nano-bacteria hybrids for efficient tumor therapy. Coord. Chem. Rev. 488, 215178 (2023). https://doi.org/10.1016/j.ccr.2023.215178
X. He, Y. Lv, Y. Lin, H. Yu, Y. Zhang et al., Platinum nanops regulated V2C MXene nanoplatforms with NIR-II enhanced nanozyme effect for photothermal and chemodynamic anti-infective therapy. Adv. Mater. 36(25), 2400366 (2024). https://doi.org/10.1002/adma.202400366
G. Kresse, J. Hafner, Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B 48(17), 13115–13118 (1993). https://doi.org/10.1103/physrevb.48.13115
J.P. Perdew, K. Burke, M. Ernzerhof, Generalized gradient approximation made simple. Phys. Rev. Lett. 77(18), 3865–3868 (1996). https://doi.org/10.1103/physrevlett.77.3865
P.E. Blöchl, Projector augmented-wave method. Phys. Rev. B 50(24), 17953–17979 (1994). https://doi.org/10.1103/physrevb.50.17953
M. Zhang, Y. Wang, C. Miao, S. Lin, Y. Zheng et al., Dextran guanidinylated carbon dots with antibacterial and immunomodulatory activities as eye drops for the topical treatment of MRSA-induced infectious keratitis. Acta Biomater. 200, 591–609 (2025). https://doi.org/10.1016/j.actbio.2025.05.032