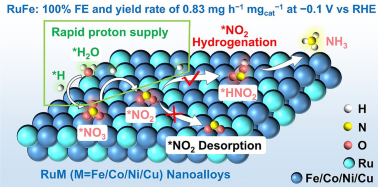
Efficient Neutral Nitrate-to-Ammonia Electrosynthesis Using Synergistic Ru-Based Nanoalloys on Nitrogen-Doped Carbon
Corresponding Author: Liang Wang
Nano-Micro Letters,
Vol. 18 (2026), Article Number: 66
Abstract
Electrocatalytic nitrate reduction reaction (NO3RR) represents a sustainable and environmentally benign route for ammonia (NH3) synthesis. However, NO3RR is still limited by the competition from hydrogen evolution reaction (HER) and the high energy barrier in the hydrogenation step of nitrogen-containing intermediates. Here, we report a selective etching strategy to construct RuM nanoalloys (M = Fe, Co, Ni, Cu) uniformly dispersed on porous nitrogen-doped carbon substrates for efficient neutral NH3 electrosynthesis. Density functional theory calculations confirm that the synergic effect between Ru and transition metal M modulates the electronic structure of the alloy, significantly lowering the energy barrier for the conversion of *NO2 to *HNO2. Experimentally, the optimized RuFe-NC catalyst achieves 100% Faraday efficiency with a high yield rate of 0.83 mg h−1 mgcat−1 at a low potential of − 0.1 V vs. RHE, outperforming most reported catalysts. In situ spectroscopic analyses further demonstrate that the RuM-NC effectively promotes the hydrogenation of nitrogen intermediates while inhibiting the formation of hydrogen radicals, thereby reducing HER competition. The RuFe-NC assembled Zn-NO3− battery achieved a high open-circuit voltage and an outstanding power density and capacity, which drive selective NO3− conversion to NH3. This work provides a powerful synergistic design strategy for efficient NH3 electrosynthesis and a general framework for the development of advanced multi-component catalysts for sustainable nitrogen conversion.
Highlights:
1 A selective etching strategy was developed to construct a serious of RuM nanoalloys (M = Fe, Co, Ni, Cu) uniformly dispersed on porous nitrogen-doped carbon.
2 It has been demonstrated that RuM nanoalloys would present the enhancement synergic effect on significantly improve the kinetic of *NO2 conversion to *HNO2, which achieves efficient neutral NH3 electrosynthesis at more positive potential.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- H. Zhang, C. Wang, H. Luo, J. Chen, M. Kuang et al., Iron nanops protected by chainmail-structured graphene for durable electrocatalytic nitrate reduction to nitrogen. Angew. Chem. Int. Ed. 62(5), e202217071 (2023). https://doi.org/10.1002/anie.202217071
- Y. Yu, Y. Li, Y. Fang, L. Wen, B. Tu et al., Recent advances of ammonia synthesis under ambient conditions over metal-organic framework based electrocatalysts. Appl. Catal. B Environ. 340, 123161 (2024). https://doi.org/10.1016/j.apcatb.2023.123161
- X. Dai, Z.-Y. Du, Y. Sun, P. Chen, X. Duan et al., Enhancing green ammonia electrosynthesis through tuning Sn vacancies in Sn-based MXene/MAX hybrids. Nano-Micro Lett. 16(1), 89 (2024). https://doi.org/10.1007/s40820-023-01303-2
- L. Hu, H.S. Pillai, C. Feit, K. Shi, Z. Gao et al., Identification of active sites for ammonia electrosynthesis on ruthenium. ACS Energy Lett. 7(12), 4290–4298 (2022). https://doi.org/10.1021/acsenergylett.2c02175
- Y. Jiang, M. Wang, L. Zhang, S. Liu, Y. Cao et al., Distorted spinel ferrite heterostructure triggered by alkaline earth metal substitution facilitates nitrogen localization and electrocatalytic reduction to ammonia. Chem. Eng. J. 450, 138226 (2022). https://doi.org/10.1016/j.cej.2022.138226
- M. Wang, M.A. Khan, I. Mohsin, J. Wicks, A.H. Ip et al., Can sustainable ammonia synthesis pathways compete with fossil-fuel based Haber-Bosch processes? Energy Environ. Sci. 14(5), 2535–2548 (2021). https://doi.org/10.1039/D0EE03808C
- J.-H. Kim, T.-Y. Dai, M. Yang, J.-M. Seo, J.S. Lee et al., Achieving volatile potassium promoted ammonia synthesis via mechanochemistry. Nat. Commun. 14(1), 2319 (2023). https://doi.org/10.1038/s41467-023-38050-2
- D.-X. Liu, Z. Meng, Y.-F. Zhu, X.-F. Sun, X. Deng et al., Gram-level NH3 electrosynthesis via NOx reduction on a Cu activated co electrode. Angew. Chem. Int. Ed. 63(1), e202315238 (2024). https://doi.org/10.1002/anie.202315238
- J. Lim, C.A. Fernández, S.W. Lee, M.C. Hatzell, Ammonia and nitric acid demands for fertilizer use in 2050. ACS Energy Lett. 6(10), 3676–3685 (2021). https://doi.org/10.1021/acsenergylett.1c01614
- Q. Zhang, C. Ni, N. Deng, X. Huang, Tailoring electronic and morphology features of iron-doped Ni2P nanoflowers for enhanced ammonia electrosynthesis in solid electrolyte reactors. Adv. Energy Mater. 15(23), 2405442 (2025). https://doi.org/10.1002/aenm.202405442
- Y. Zhou, L. Zhang, Z. Zhu, M. Wang, N. Li et al., Optimizing intermediate adsorption over PdM (M=Fe Co, Ni, Cu) bimetallene for boosted nitrate electroreduction to ammonia. Angew. Chem. Int. Ed. 63(18), e202319029 (2024). https://doi.org/10.1002/anie.202319029
- S. Zhang, Y. Zha, Y. Ye, K. Li, Y. Lin et al., Oxygen-coordinated single Mn sites for efficient electrocatalytic nitrate reduction to ammonia. Nano-Micro Lett. 16(1), 9 (2023). https://doi.org/10.1007/s40820-023-01217-z
- L.-H. Zhang, Y. Jia, J. Zhan, G. Liu, G. Liu et al., Dopant-induced electronic states regulation boosting electroreduction of dilute nitrate to ammonium. Angew. Chem. Int. Ed. 62(22), e202303483 (2023). https://doi.org/10.1002/anie.202303483
- Y. Wang, M. Sun, J. Zhou, Y. Xiong, Q. Zhang et al., Atomic coordination environment engineering of bimetallic alloy nanostructures for efficient ammonia electrosynthesis from nitrate. Proc. Natl. Acad. Sci. U. S. A. 120(32), e2306461120 (2023). https://doi.org/10.1073/pnas.2306461120
- J. Li, G. Zhan, J. Yang, F. Quan, C. Mao et al., Efficient ammonia electrosynthesis from nitrate on strained ruthenium nanoclusters. J. Am. Chem. Soc. 142(15), 7036–7046 (2020). https://doi.org/10.1021/jacs.0c00418
- P. Li, R. Li, Y. Liu, M. Xie, Z. Jin et al., Pulsed nitrate-to-ammonia electroreduction facilitated by tandem catalysis of nitrite intermediates. J. Am. Chem. Soc. 145(11), 6471–6479 (2023). https://doi.org/10.1021/jacs.3c00334
- X. Long, F. Huang, T. Zhong, H. Zhao, P. Li et al., One-step strategy to maximize single-atom catalyst utilization in nitrate reduction via bidirectional optimization of mass transfer and electron supply. Environ. Sci. Technol. 59(17), 8555–8567 (2025). https://doi.org/10.1021/acs.est.4c14011
- X.-F. Cheng, J.-H. He, H.-Q. Ji, H.-Y. Zhang, Q. Cao et al., Coordination symmetry breaking of single-atom catalysts for robust and efficient nitrate electroreduction to ammonia. Adv. Mater. 34(36), 2205767 (2022). https://doi.org/10.1002/adma.202205767
- C. Wu, Y. Shen, L. Lv, X. Meng, X. Yang et al., Multi-orbital engineering of single-atom catalysts: unlocking high-efficiency nitrate reduction. J. Mater. Chem. A. 13(9), 6631–6643 (2025). https://doi.org/10.1039/d4ta08757g
- Y. Zhou, R. Duan, H. Li, M. Zhao, C. Ding et al., Boosting electrocatalytic nitrate reduction to ammonia via promoting water dissociation. ACS Catal. 13(16), 10846–10854 (2023). https://doi.org/10.1021/acscatal.3c02951
- Y. Xiong, M. Sun, S. Wang, Y. Wang, J. Zhou et al., Atomic scale cooperativity of alloy nanostructures for efficient nitrate electroreduction to ammonia in neutral media. Adv. Funct. Mater. 35(14), 2420153 (2025). https://doi.org/10.1002/adfm.202420153
- X. Ge, R. Pan, H. Xie, S. Hu, J. Yuan, Regulating RuxMoy nanoalloys anchored on porous nitrogen-doped carbon via domain-confined etching strategy for neutral efficient ammonia electrosynthesis. Nano Lett. 24(39), 12218–12225 (2024). https://doi.org/10.1021/acs.nanolett.4c03319
- Y. Wang, F. Hao, H. Xu, M. Sun, X. Wang et al., Interfacial water structure modulation on unconventional phase non-precious metal alloy nanostructures for efficient nitrate electroreduction to ammonia in neutral media. Angew. Chem. Int. Ed. 64(28), e202508617 (2025). https://doi.org/10.1002/anie.202508617
- Y. Wang, Y. Xiong, M. Sun, J. Zhou, F. Hao et al., Controlled synthesis of unconventional phase alloy nanobranches for highly selective electrocatalytic nitrite reduction to ammonia. Angew. Chem. Int. Ed. 63(26), e202402841 (2024). https://doi.org/10.1002/anie.202402841
- Y. Xiong, Y. Wang, M. Sun, J. Chen, J. Zhou et al., Regulating the electrochemical nitrate reduction performance with controllable distribution of unconventional phase copper on alloy nanostructures. Adv. Mater. 36(45), 2407889 (2024). https://doi.org/10.1002/adma.202407889
- H. Zhang, C. Ma, Y.-C. Wang, X. Zhu, K. Qu et al., Transition metal-gallium intermetallic compounds with tailored active site configurations for electrochemical ammonia synthesis. Angew. Chem. Int. Ed. 63(49), e202409515 (2024). https://doi.org/10.1002/anie.202409515
- Y. Wang, S. Qin, X. Chen, X. Meng, Z. Li, Fe-modified Co2Mo3O8-promoted nitrate-cascade reduction reaction coupled with the oxygen evolution reaction for electrocatalytic ammonia synthesis. Inorg. Chem. Front. 11(18), 6052–6063 (2024). https://doi.org/10.1039/D4QI01465K
- Y. Wang, L. Zhang, Y. Niu, D. Fang, J. Wang et al., Boosting NH3 production from nitrate electroreduction via electronic structure engineering of Fe3C nanoflakes. Green Chem. 23(19), 7594–7608 (2021). https://doi.org/10.1039/D1GC01913A
- M. Liu, Z. Lu, L. Yang, R. Gao, X. Zhang et al., Co-N bond promotes the H* pathway for the electrocatalytic reduction of nitrate (NO3RR) to ammonia. J. Environ. Chem. Eng. 11(3), 109718 (2023). https://doi.org/10.1016/j.jece.2023.109718
- X. Sun, Y. He, M. Wang, Q. Cheng, Y. Huan et al., Maximizing available active hydrogen on FeNi substitutional solid-solution alloy to boost electrosynthesis of ammonia from nitrate. ACS Nano. 19(8), 8189–8199 (2025). https://doi.org/10.1021/acsnano.4c17163
- J. Guan, L. Cai, W. Li, H. Zhou, Y. Huang, Boosting nitrate electroreduction to ammonia on atomic Ru-Co pair sites in hollow spinels. Appl. Catal. B Environ. Energy. 358, 124387 (2024). https://doi.org/10.1016/j.apcatb.2024.124387
- X. Ouyang, W. Qiao, Y. Yang, B. Xi, Y. Yu et al., Intensifying interfacial reverse hydrogen spillover for boosted electrocatalytic nitrate reduction to ammonia. Angew. Chem. Int. Ed. 137(13), e202422585 (2025). https://doi.org/10.1002/ange.202422585
- F. Liu, J. Zhou, M. Sun, Z. Xu, H. Wang et al., Enhanced p–d orbital coupling in unconventional phase RhSb alloy nanoflowers for efficient ammonia electrosynthesis in neutral media. Angew. Chem. Int. Ed. 64(23), e202504641 (2025). https://doi.org/10.1002/anie.202504641
- J. Zhang, Y. Liu, J. Zhang, J. Guan, H. Ke et al., Engineering single-atomic Ru sites on cobalt hydroxide boosts atomic hydrogen generation for efficient nitrate electroreduction to ammonia. Renewables. 3(2), 99–110 (2025). https://doi.org/10.31635/renewables.025.202500086
- G. Kresse, J. Hafner, Ab initiomolecular dynamics for liquid metals. Phys. Rev. B 47(1), 558–561 (1993). https://doi.org/10.1103/physrevb.47.558
- J.P. Perdew, K. Burke, M. Ernzerhof, Generalized gradient approximation made simple. Phys. Rev. Lett. 77(18), 3865–3868 (1996). https://doi.org/10.1103/physrevlett.77.3865
- P.E. Blöchl, Projector augmented-wave method. Phys. Rev. B. 50(24), 17953–17979 (1994). https://doi.org/10.1103/physrevb.50.17953
- H.J. Monkhorst, J.D. Pack, Special points for Brillouin-zone integrations. Phys. Rev. B 13(12), 5188–5192 (1976). https://doi.org/10.1103/physrevb.13.5188
- S. Grimme, J. Antony, S. Ehrlich, H. Krieg, A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132(15), 154104 (2010). https://doi.org/10.1063/1.3382344
- E. Skúlason, V. Tripkovic, M.E. Björketun, S. Gudmundsdóttir, G. Karlberg et al., Modeling the electrochemical hydrogen oxidation and evolution reactions on the basis of density functional theory calculations. J. Phys. Chem. C. 114(50), 22374–22374 (2010). https://doi.org/10.1021/jp110913n
- L. Wu, J. Feng, L. Zhang, S. Jia, X. Song et al., Boosting electrocatalytic nitrate-to-ammonia via tuning of N-intermediate adsorption on a Zn−Cu catalyst. Angew. Chem. Int. Ed. 62(43), e202307952 (2023). https://doi.org/10.1002/anie.202307952
- M. Xie, S. Tang, Z. Li, M. Wang, Z. Jin et al., Intermetallic single-atom alloy In–Pd bimetallene for neutral electrosynthesis of ammonia from nitrate. J. Am. Chem. Soc. 145(25), 13957–13967 (2023). https://doi.org/10.1021/jacs.3c03432
- K. Chu, W. Zong, G. Xue, H. Guo, J. Qin et al., Cation substitution strategy for developing perovskite oxide with rich oxygen vacancy-mediated charge redistribution enables highly efficient nitrate electroreduction to ammonia. J. Am. Chem. Soc. 145(39), 21387–21396 (2023). https://doi.org/10.1021/jacs.3c06402
- J. Chen, Y. Ha, R. Wang, Y. Liu, H. Xu et al., Inner co synergizing outer Ru supported on carbon nanotubes for efficient pH-universal hydrogen evolution catalysis. Nano-Micro Lett. 14(1), 186 (2022). https://doi.org/10.1007/s40820-022-00933-2
- J. He, Y. Zhao, Y. Li, Q. Yuan, Y. Wu et al., Joule heating-driven sp-C domains modulation in biomass carbon for high-performance bifunctional oxygen electrocatalysis. Nano-Micro Lett. 17(1), 221 (2025). https://doi.org/10.1007/s40820-025-01725-0
- Y. Huang, C. Zhang, X. Wang, Y. Wu, J. Lv et al., Synergistic single-atom and clustered cobalt sites on N/S Co-doped defect nano-carbon for efficient H2O2 electrosynthesis. Nano-Micro Lett. 17(1), 142 (2025). https://doi.org/10.1007/s40820-025-01657-9
- H. Yin, J. Yuan, J. Wang, S. Hu, P. Wang et al., Crystalline nitrogen-doped-carbon anchored well-dispersed Fe3O4 nanops for real-time scalable neutral H2O2 electrosynthesis. Energy Environ. Sci. 18(5), 2231–2242 (2025). https://doi.org/10.1039/D4EE05796A
- P. Zhang, S. Huang, K. Chen, X. Liu, Y. Xu et al., Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction. Nano-Micro Lett. 17(1), 278 (2025). https://doi.org/10.1007/s40820-025-01783-4
- Y. Liu, X. Tu, X. Wei, D. Wang, X. Zhang et al., C-bound or O-bound surface: which one boosts electrocatalytic urea synthesis? Angew. Chem. Int. Ed. 62(19), e202300387 (2023). https://doi.org/10.1002/anie.202300387
- G. An, K. Wang, M. Yang, J. Zhang, H. Zhong et al., Rational design of metal-free nitrogen-doped carbon for controllable reduction of CO2 to syngas. Molecules. 30(4), 953 (2025). https://doi.org/10.3390/molecules30040953
- W. Liu, B. Tang, K. Huang, Z. Zhang, Z. Wang et al., Radiation-synthesized metal–organic frameworks with ligand-induced lewis pairs for selective CO2 electroreduction. Small. 20(52), 2408688 (2024). https://doi.org/10.1002/smll.202408688
- J. Xiong, L. Jiang, B. Zhu, S. Huang, S. Wu et al., FeIr alloy optimizes the trade-off between nitrate reduction and active hydrogen generation for efficient electro-synthesis of ammonia in neutral media. Adv. Funct. Mater. 35(27), 2423705 (2025). https://doi.org/10.1002/adfm.202423705
- J. Zhou, Y. Xiong, M. Sun, Z. Xu, Y. Wang et al., Constructing molecule-metal relay catalysis over heterophase metallene for high-performance rechargeable zinc-nitrate/ethanol batteries. Proc. Natl. Acad. Sci. U. S. A. 120(50), e2311149120 (2023). https://doi.org/10.1073/pnas.2311149120
- C. Yang, H. Pang, X. Li, X. Zheng, T. Wei et al., Scalable electrocatalytic urea wastewater treatment coupled with hydrogen production by regulating adsorption behavior of urea molecule. Nano-Micro Lett. 17(1), 159 (2025). https://doi.org/10.1007/s40820-024-01585-0
- K. Wang, K. Huang, Z. Wang, G. An, M. Zhang et al., Functional group engineering of single-walled carbon nanotubes for anchoring copper nanops toward selective CO2 electroreduction to C2 products. Small. 21(21), 2502733 (2025). https://doi.org/10.1002/smll.202502733
- Z. Wang, H. Guo, S. Zhang, J. Zhang, L. Wang, Highly selective electrosynthesis of hydrogen peroxide with tellurium nanops encapsulated in ultrathin graphitic carbon shells. Chem. Eng. J. 512, 162752 (2025). https://doi.org/10.1016/j.cej.2025.162752
- G. An, K. Wang, Z. Wang, M. Zhang, H. Guo et al., Amine-functionalized metal-free nanocarbon to boost selective CO2 electroreduction to CO in a flow cell. ACS Appl. Mater. Interfaces 16(22), 29060–29068 (2024). https://doi.org/10.1021/acsami.4c04502
- W. Gao, K. Xie, J. Xie, X. Wang, H. Zhang et al., Alloying of Cu with Ru enabling the relay catalysis for reduction of nitrate to ammonia. Adv. Mater. 35(19), 2202952 (2023). https://doi.org/10.1002/adma.202202952
- J.-Y. Fang, Q.-Z. Zheng, Y.-Y. Lou, K.-M. Zhao, S.-N. Hu et al., Ampere-level current density ammonia electrochemical synthesis using CuCo nanosheets simulating nitrite reductase bifunctional nature. Nat. Commun. 13(1), 7899 (2022). https://doi.org/10.1038/s41467-022-35533-6
- N.C. Kani, N.H.L. Nguyen, K. Markel, R.R. Bhawnani, B. Shindel et al., Electrochemical reduction of nitrates on CoO nanoclusters-functionalized graphene with highest mass activity and nearly 100% selectivity to ammonia. Adv. Energy Mater. 13(17), 2204236 (2023). https://doi.org/10.1002/aenm.202204236
- A. Guiet, A. Simonin, H. Bemana, H. Al-Mahayni, J. Li et al., Reversible transition of an amorphous Cu-Al oxyfluoride into a highly active electrocatalyst for NO3–reduction to NH3. Chem Catal. 3(5), 100595 (2023). https://doi.org/10.1016/j.checat.2023.100595
- J. Wang, H.T.D. Bui, H. Hu, S. Kong, X. Wang et al., Industrial-current ammonia synthesis by polarized cuprous cyanamide coupled to valorization of glycerol at 4,000 mA cm−2. Adv. Mater. 37(14), 2418451 (2025). https://doi.org/10.1002/adma.202418451
- B. Zhou, L. Yu, W. Zhang, X. Liu, H. Zhang et al., Cu1−Fe dual sites for superior neutral ammonia electrosynthesis from nitrate. Angew. Chem. Int. Ed. 63(31), e202406046 (2024). https://doi.org/10.1002/anie.202406046
- S. Liang, X. Teng, H. Xu, L. Chen, J. Shi, H* species regulation by Mn-Co(OH)2 for efficient nitrate electro-reduction in neutral solution. Angew. Chem. Int. Ed. 63(11), e202400206 (2024). https://doi.org/10.1002/anie.202400206
- W. Chen, Y. Zhang, M. Yang, C. Yang, Z. Meng, Single-point linkage engineering in conjugated phthalocyanine-based covalent organic frameworks for electrochemical CO2 reduction. Nano-Micro Lett. 17(1), 252 (2025). https://doi.org/10.1007/s40820-025-01754-9
References
H. Zhang, C. Wang, H. Luo, J. Chen, M. Kuang et al., Iron nanops protected by chainmail-structured graphene for durable electrocatalytic nitrate reduction to nitrogen. Angew. Chem. Int. Ed. 62(5), e202217071 (2023). https://doi.org/10.1002/anie.202217071
Y. Yu, Y. Li, Y. Fang, L. Wen, B. Tu et al., Recent advances of ammonia synthesis under ambient conditions over metal-organic framework based electrocatalysts. Appl. Catal. B Environ. 340, 123161 (2024). https://doi.org/10.1016/j.apcatb.2023.123161
X. Dai, Z.-Y. Du, Y. Sun, P. Chen, X. Duan et al., Enhancing green ammonia electrosynthesis through tuning Sn vacancies in Sn-based MXene/MAX hybrids. Nano-Micro Lett. 16(1), 89 (2024). https://doi.org/10.1007/s40820-023-01303-2
L. Hu, H.S. Pillai, C. Feit, K. Shi, Z. Gao et al., Identification of active sites for ammonia electrosynthesis on ruthenium. ACS Energy Lett. 7(12), 4290–4298 (2022). https://doi.org/10.1021/acsenergylett.2c02175
Y. Jiang, M. Wang, L. Zhang, S. Liu, Y. Cao et al., Distorted spinel ferrite heterostructure triggered by alkaline earth metal substitution facilitates nitrogen localization and electrocatalytic reduction to ammonia. Chem. Eng. J. 450, 138226 (2022). https://doi.org/10.1016/j.cej.2022.138226
M. Wang, M.A. Khan, I. Mohsin, J. Wicks, A.H. Ip et al., Can sustainable ammonia synthesis pathways compete with fossil-fuel based Haber-Bosch processes? Energy Environ. Sci. 14(5), 2535–2548 (2021). https://doi.org/10.1039/D0EE03808C
J.-H. Kim, T.-Y. Dai, M. Yang, J.-M. Seo, J.S. Lee et al., Achieving volatile potassium promoted ammonia synthesis via mechanochemistry. Nat. Commun. 14(1), 2319 (2023). https://doi.org/10.1038/s41467-023-38050-2
D.-X. Liu, Z. Meng, Y.-F. Zhu, X.-F. Sun, X. Deng et al., Gram-level NH3 electrosynthesis via NOx reduction on a Cu activated co electrode. Angew. Chem. Int. Ed. 63(1), e202315238 (2024). https://doi.org/10.1002/anie.202315238
J. Lim, C.A. Fernández, S.W. Lee, M.C. Hatzell, Ammonia and nitric acid demands for fertilizer use in 2050. ACS Energy Lett. 6(10), 3676–3685 (2021). https://doi.org/10.1021/acsenergylett.1c01614
Q. Zhang, C. Ni, N. Deng, X. Huang, Tailoring electronic and morphology features of iron-doped Ni2P nanoflowers for enhanced ammonia electrosynthesis in solid electrolyte reactors. Adv. Energy Mater. 15(23), 2405442 (2025). https://doi.org/10.1002/aenm.202405442
Y. Zhou, L. Zhang, Z. Zhu, M. Wang, N. Li et al., Optimizing intermediate adsorption over PdM (M=Fe Co, Ni, Cu) bimetallene for boosted nitrate electroreduction to ammonia. Angew. Chem. Int. Ed. 63(18), e202319029 (2024). https://doi.org/10.1002/anie.202319029
S. Zhang, Y. Zha, Y. Ye, K. Li, Y. Lin et al., Oxygen-coordinated single Mn sites for efficient electrocatalytic nitrate reduction to ammonia. Nano-Micro Lett. 16(1), 9 (2023). https://doi.org/10.1007/s40820-023-01217-z
L.-H. Zhang, Y. Jia, J. Zhan, G. Liu, G. Liu et al., Dopant-induced electronic states regulation boosting electroreduction of dilute nitrate to ammonium. Angew. Chem. Int. Ed. 62(22), e202303483 (2023). https://doi.org/10.1002/anie.202303483
Y. Wang, M. Sun, J. Zhou, Y. Xiong, Q. Zhang et al., Atomic coordination environment engineering of bimetallic alloy nanostructures for efficient ammonia electrosynthesis from nitrate. Proc. Natl. Acad. Sci. U. S. A. 120(32), e2306461120 (2023). https://doi.org/10.1073/pnas.2306461120
J. Li, G. Zhan, J. Yang, F. Quan, C. Mao et al., Efficient ammonia electrosynthesis from nitrate on strained ruthenium nanoclusters. J. Am. Chem. Soc. 142(15), 7036–7046 (2020). https://doi.org/10.1021/jacs.0c00418
P. Li, R. Li, Y. Liu, M. Xie, Z. Jin et al., Pulsed nitrate-to-ammonia electroreduction facilitated by tandem catalysis of nitrite intermediates. J. Am. Chem. Soc. 145(11), 6471–6479 (2023). https://doi.org/10.1021/jacs.3c00334
X. Long, F. Huang, T. Zhong, H. Zhao, P. Li et al., One-step strategy to maximize single-atom catalyst utilization in nitrate reduction via bidirectional optimization of mass transfer and electron supply. Environ. Sci. Technol. 59(17), 8555–8567 (2025). https://doi.org/10.1021/acs.est.4c14011
X.-F. Cheng, J.-H. He, H.-Q. Ji, H.-Y. Zhang, Q. Cao et al., Coordination symmetry breaking of single-atom catalysts for robust and efficient nitrate electroreduction to ammonia. Adv. Mater. 34(36), 2205767 (2022). https://doi.org/10.1002/adma.202205767
C. Wu, Y. Shen, L. Lv, X. Meng, X. Yang et al., Multi-orbital engineering of single-atom catalysts: unlocking high-efficiency nitrate reduction. J. Mater. Chem. A. 13(9), 6631–6643 (2025). https://doi.org/10.1039/d4ta08757g
Y. Zhou, R. Duan, H. Li, M. Zhao, C. Ding et al., Boosting electrocatalytic nitrate reduction to ammonia via promoting water dissociation. ACS Catal. 13(16), 10846–10854 (2023). https://doi.org/10.1021/acscatal.3c02951
Y. Xiong, M. Sun, S. Wang, Y. Wang, J. Zhou et al., Atomic scale cooperativity of alloy nanostructures for efficient nitrate electroreduction to ammonia in neutral media. Adv. Funct. Mater. 35(14), 2420153 (2025). https://doi.org/10.1002/adfm.202420153
X. Ge, R. Pan, H. Xie, S. Hu, J. Yuan, Regulating RuxMoy nanoalloys anchored on porous nitrogen-doped carbon via domain-confined etching strategy for neutral efficient ammonia electrosynthesis. Nano Lett. 24(39), 12218–12225 (2024). https://doi.org/10.1021/acs.nanolett.4c03319
Y. Wang, F. Hao, H. Xu, M. Sun, X. Wang et al., Interfacial water structure modulation on unconventional phase non-precious metal alloy nanostructures for efficient nitrate electroreduction to ammonia in neutral media. Angew. Chem. Int. Ed. 64(28), e202508617 (2025). https://doi.org/10.1002/anie.202508617
Y. Wang, Y. Xiong, M. Sun, J. Zhou, F. Hao et al., Controlled synthesis of unconventional phase alloy nanobranches for highly selective electrocatalytic nitrite reduction to ammonia. Angew. Chem. Int. Ed. 63(26), e202402841 (2024). https://doi.org/10.1002/anie.202402841
Y. Xiong, Y. Wang, M. Sun, J. Chen, J. Zhou et al., Regulating the electrochemical nitrate reduction performance with controllable distribution of unconventional phase copper on alloy nanostructures. Adv. Mater. 36(45), 2407889 (2024). https://doi.org/10.1002/adma.202407889
H. Zhang, C. Ma, Y.-C. Wang, X. Zhu, K. Qu et al., Transition metal-gallium intermetallic compounds with tailored active site configurations for electrochemical ammonia synthesis. Angew. Chem. Int. Ed. 63(49), e202409515 (2024). https://doi.org/10.1002/anie.202409515
Y. Wang, S. Qin, X. Chen, X. Meng, Z. Li, Fe-modified Co2Mo3O8-promoted nitrate-cascade reduction reaction coupled with the oxygen evolution reaction for electrocatalytic ammonia synthesis. Inorg. Chem. Front. 11(18), 6052–6063 (2024). https://doi.org/10.1039/D4QI01465K
Y. Wang, L. Zhang, Y. Niu, D. Fang, J. Wang et al., Boosting NH3 production from nitrate electroreduction via electronic structure engineering of Fe3C nanoflakes. Green Chem. 23(19), 7594–7608 (2021). https://doi.org/10.1039/D1GC01913A
M. Liu, Z. Lu, L. Yang, R. Gao, X. Zhang et al., Co-N bond promotes the H* pathway for the electrocatalytic reduction of nitrate (NO3RR) to ammonia. J. Environ. Chem. Eng. 11(3), 109718 (2023). https://doi.org/10.1016/j.jece.2023.109718
X. Sun, Y. He, M. Wang, Q. Cheng, Y. Huan et al., Maximizing available active hydrogen on FeNi substitutional solid-solution alloy to boost electrosynthesis of ammonia from nitrate. ACS Nano. 19(8), 8189–8199 (2025). https://doi.org/10.1021/acsnano.4c17163
J. Guan, L. Cai, W. Li, H. Zhou, Y. Huang, Boosting nitrate electroreduction to ammonia on atomic Ru-Co pair sites in hollow spinels. Appl. Catal. B Environ. Energy. 358, 124387 (2024). https://doi.org/10.1016/j.apcatb.2024.124387
X. Ouyang, W. Qiao, Y. Yang, B. Xi, Y. Yu et al., Intensifying interfacial reverse hydrogen spillover for boosted electrocatalytic nitrate reduction to ammonia. Angew. Chem. Int. Ed. 137(13), e202422585 (2025). https://doi.org/10.1002/ange.202422585
F. Liu, J. Zhou, M. Sun, Z. Xu, H. Wang et al., Enhanced p–d orbital coupling in unconventional phase RhSb alloy nanoflowers for efficient ammonia electrosynthesis in neutral media. Angew. Chem. Int. Ed. 64(23), e202504641 (2025). https://doi.org/10.1002/anie.202504641
J. Zhang, Y. Liu, J. Zhang, J. Guan, H. Ke et al., Engineering single-atomic Ru sites on cobalt hydroxide boosts atomic hydrogen generation for efficient nitrate electroreduction to ammonia. Renewables. 3(2), 99–110 (2025). https://doi.org/10.31635/renewables.025.202500086
G. Kresse, J. Hafner, Ab initiomolecular dynamics for liquid metals. Phys. Rev. B 47(1), 558–561 (1993). https://doi.org/10.1103/physrevb.47.558
J.P. Perdew, K. Burke, M. Ernzerhof, Generalized gradient approximation made simple. Phys. Rev. Lett. 77(18), 3865–3868 (1996). https://doi.org/10.1103/physrevlett.77.3865
P.E. Blöchl, Projector augmented-wave method. Phys. Rev. B. 50(24), 17953–17979 (1994). https://doi.org/10.1103/physrevb.50.17953
H.J. Monkhorst, J.D. Pack, Special points for Brillouin-zone integrations. Phys. Rev. B 13(12), 5188–5192 (1976). https://doi.org/10.1103/physrevb.13.5188
S. Grimme, J. Antony, S. Ehrlich, H. Krieg, A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132(15), 154104 (2010). https://doi.org/10.1063/1.3382344
E. Skúlason, V. Tripkovic, M.E. Björketun, S. Gudmundsdóttir, G. Karlberg et al., Modeling the electrochemical hydrogen oxidation and evolution reactions on the basis of density functional theory calculations. J. Phys. Chem. C. 114(50), 22374–22374 (2010). https://doi.org/10.1021/jp110913n
L. Wu, J. Feng, L. Zhang, S. Jia, X. Song et al., Boosting electrocatalytic nitrate-to-ammonia via tuning of N-intermediate adsorption on a Zn−Cu catalyst. Angew. Chem. Int. Ed. 62(43), e202307952 (2023). https://doi.org/10.1002/anie.202307952
M. Xie, S. Tang, Z. Li, M. Wang, Z. Jin et al., Intermetallic single-atom alloy In–Pd bimetallene for neutral electrosynthesis of ammonia from nitrate. J. Am. Chem. Soc. 145(25), 13957–13967 (2023). https://doi.org/10.1021/jacs.3c03432
K. Chu, W. Zong, G. Xue, H. Guo, J. Qin et al., Cation substitution strategy for developing perovskite oxide with rich oxygen vacancy-mediated charge redistribution enables highly efficient nitrate electroreduction to ammonia. J. Am. Chem. Soc. 145(39), 21387–21396 (2023). https://doi.org/10.1021/jacs.3c06402
J. Chen, Y. Ha, R. Wang, Y. Liu, H. Xu et al., Inner co synergizing outer Ru supported on carbon nanotubes for efficient pH-universal hydrogen evolution catalysis. Nano-Micro Lett. 14(1), 186 (2022). https://doi.org/10.1007/s40820-022-00933-2
J. He, Y. Zhao, Y. Li, Q. Yuan, Y. Wu et al., Joule heating-driven sp-C domains modulation in biomass carbon for high-performance bifunctional oxygen electrocatalysis. Nano-Micro Lett. 17(1), 221 (2025). https://doi.org/10.1007/s40820-025-01725-0
Y. Huang, C. Zhang, X. Wang, Y. Wu, J. Lv et al., Synergistic single-atom and clustered cobalt sites on N/S Co-doped defect nano-carbon for efficient H2O2 electrosynthesis. Nano-Micro Lett. 17(1), 142 (2025). https://doi.org/10.1007/s40820-025-01657-9
H. Yin, J. Yuan, J. Wang, S. Hu, P. Wang et al., Crystalline nitrogen-doped-carbon anchored well-dispersed Fe3O4 nanops for real-time scalable neutral H2O2 electrosynthesis. Energy Environ. Sci. 18(5), 2231–2242 (2025). https://doi.org/10.1039/D4EE05796A
P. Zhang, S. Huang, K. Chen, X. Liu, Y. Xu et al., Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction. Nano-Micro Lett. 17(1), 278 (2025). https://doi.org/10.1007/s40820-025-01783-4
Y. Liu, X. Tu, X. Wei, D. Wang, X. Zhang et al., C-bound or O-bound surface: which one boosts electrocatalytic urea synthesis? Angew. Chem. Int. Ed. 62(19), e202300387 (2023). https://doi.org/10.1002/anie.202300387
G. An, K. Wang, M. Yang, J. Zhang, H. Zhong et al., Rational design of metal-free nitrogen-doped carbon for controllable reduction of CO2 to syngas. Molecules. 30(4), 953 (2025). https://doi.org/10.3390/molecules30040953
W. Liu, B. Tang, K. Huang, Z. Zhang, Z. Wang et al., Radiation-synthesized metal–organic frameworks with ligand-induced lewis pairs for selective CO2 electroreduction. Small. 20(52), 2408688 (2024). https://doi.org/10.1002/smll.202408688
J. Xiong, L. Jiang, B. Zhu, S. Huang, S. Wu et al., FeIr alloy optimizes the trade-off between nitrate reduction and active hydrogen generation for efficient electro-synthesis of ammonia in neutral media. Adv. Funct. Mater. 35(27), 2423705 (2025). https://doi.org/10.1002/adfm.202423705
J. Zhou, Y. Xiong, M. Sun, Z. Xu, Y. Wang et al., Constructing molecule-metal relay catalysis over heterophase metallene for high-performance rechargeable zinc-nitrate/ethanol batteries. Proc. Natl. Acad. Sci. U. S. A. 120(50), e2311149120 (2023). https://doi.org/10.1073/pnas.2311149120
C. Yang, H. Pang, X. Li, X. Zheng, T. Wei et al., Scalable electrocatalytic urea wastewater treatment coupled with hydrogen production by regulating adsorption behavior of urea molecule. Nano-Micro Lett. 17(1), 159 (2025). https://doi.org/10.1007/s40820-024-01585-0
K. Wang, K. Huang, Z. Wang, G. An, M. Zhang et al., Functional group engineering of single-walled carbon nanotubes for anchoring copper nanops toward selective CO2 electroreduction to C2 products. Small. 21(21), 2502733 (2025). https://doi.org/10.1002/smll.202502733
Z. Wang, H. Guo, S. Zhang, J. Zhang, L. Wang, Highly selective electrosynthesis of hydrogen peroxide with tellurium nanops encapsulated in ultrathin graphitic carbon shells. Chem. Eng. J. 512, 162752 (2025). https://doi.org/10.1016/j.cej.2025.162752
G. An, K. Wang, Z. Wang, M. Zhang, H. Guo et al., Amine-functionalized metal-free nanocarbon to boost selective CO2 electroreduction to CO in a flow cell. ACS Appl. Mater. Interfaces 16(22), 29060–29068 (2024). https://doi.org/10.1021/acsami.4c04502
W. Gao, K. Xie, J. Xie, X. Wang, H. Zhang et al., Alloying of Cu with Ru enabling the relay catalysis for reduction of nitrate to ammonia. Adv. Mater. 35(19), 2202952 (2023). https://doi.org/10.1002/adma.202202952
J.-Y. Fang, Q.-Z. Zheng, Y.-Y. Lou, K.-M. Zhao, S.-N. Hu et al., Ampere-level current density ammonia electrochemical synthesis using CuCo nanosheets simulating nitrite reductase bifunctional nature. Nat. Commun. 13(1), 7899 (2022). https://doi.org/10.1038/s41467-022-35533-6
N.C. Kani, N.H.L. Nguyen, K. Markel, R.R. Bhawnani, B. Shindel et al., Electrochemical reduction of nitrates on CoO nanoclusters-functionalized graphene with highest mass activity and nearly 100% selectivity to ammonia. Adv. Energy Mater. 13(17), 2204236 (2023). https://doi.org/10.1002/aenm.202204236
A. Guiet, A. Simonin, H. Bemana, H. Al-Mahayni, J. Li et al., Reversible transition of an amorphous Cu-Al oxyfluoride into a highly active electrocatalyst for NO3–reduction to NH3. Chem Catal. 3(5), 100595 (2023). https://doi.org/10.1016/j.checat.2023.100595
J. Wang, H.T.D. Bui, H. Hu, S. Kong, X. Wang et al., Industrial-current ammonia synthesis by polarized cuprous cyanamide coupled to valorization of glycerol at 4,000 mA cm−2. Adv. Mater. 37(14), 2418451 (2025). https://doi.org/10.1002/adma.202418451
B. Zhou, L. Yu, W. Zhang, X. Liu, H. Zhang et al., Cu1−Fe dual sites for superior neutral ammonia electrosynthesis from nitrate. Angew. Chem. Int. Ed. 63(31), e202406046 (2024). https://doi.org/10.1002/anie.202406046
S. Liang, X. Teng, H. Xu, L. Chen, J. Shi, H* species regulation by Mn-Co(OH)2 for efficient nitrate electro-reduction in neutral solution. Angew. Chem. Int. Ed. 63(11), e202400206 (2024). https://doi.org/10.1002/anie.202400206
W. Chen, Y. Zhang, M. Yang, C. Yang, Z. Meng, Single-point linkage engineering in conjugated phthalocyanine-based covalent organic frameworks for electrochemical CO2 reduction. Nano-Micro Lett. 17(1), 252 (2025). https://doi.org/10.1007/s40820-025-01754-9